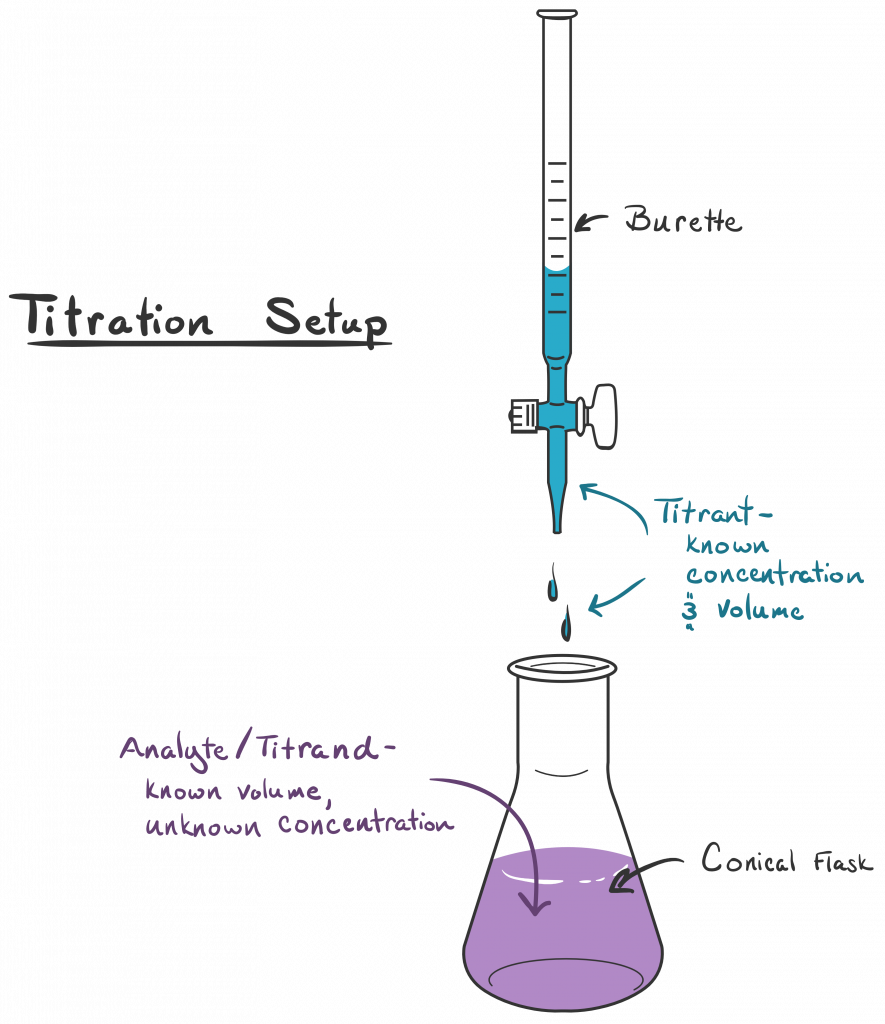
Limitations To Titration . method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. 1.1 introduction to titration. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. students who conduct a titration experiment may believe their results are as accurate as possible, but like any experiment, titration experiments contain. The lack of a strong base titrant for the. the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end.
from www.microlit.com
The lack of a strong base titrant for the. method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the. students who conduct a titration experiment may believe their results are as accurate as possible, but like any experiment, titration experiments contain. 1.1 introduction to titration.
An Advanced Guide to Titration Microlit
Limitations To Titration method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. students who conduct a titration experiment may believe their results are as accurate as possible, but like any experiment, titration experiments contain. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The lack of a strong base titrant for the. method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the. the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. 1.1 introduction to titration.
From novapublishers.com
Titration Theory, Types, Techniques and Uses Nova Science Publishers Limitations To Titration 1.1 introduction to titration. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the. the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. students who conduct a titration experiment may believe their results are as accurate as possible, but. Limitations To Titration.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and Limitations To Titration The lack of a strong base titrant for the. students who conduct a titration experiment may believe their results are as accurate as possible, but like any experiment, titration experiments contain. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. the aim of. Limitations To Titration.
From www.youtube.com
Errors in titrations YouTube Limitations To Titration students who conduct a titration experiment may believe their results are as accurate as possible, but like any experiment, titration experiments contain. 1.1 introduction to titration. method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. A titration is a quantitative, volumetric procedure used in analytical chemistry. Limitations To Titration.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Limitations To Titration 1.1 introduction to titration. The lack of a strong base titrant for the. students who conduct a titration experiment may believe their results are as accurate as possible, but like any experiment, titration experiments contain. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Limitations To Titration.
From chem.libretexts.org
Chapter 16.6 Buffers Chemistry LibreTexts Limitations To Titration students who conduct a titration experiment may believe their results are as accurate as possible, but like any experiment, titration experiments contain. The lack of a strong base titrant for the. the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. titration is the. Limitations To Titration.
From mmerevise.co.uk
Titrations and Uncertainties MME Limitations To Titration titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. The lack of a strong base titrant for the. the aim of this. Limitations To Titration.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5970774 Limitations To Titration A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. . Limitations To Titration.
From byjus.com
What is Titration? What are its types? Explain briefly Limitations To Titration a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. students who conduct a titration experiment may believe their results are as accurate as possible, but like any experiment, titration experiments contain. 1.1 introduction to titration. the aim of this booklet is to explore titration. Limitations To Titration.
From facts.net
13 Surprising Facts About Back Titration Limitations To Titration titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,.. Limitations To Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Limitations To Titration titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,.. Limitations To Titration.
From www.scribd.com
Titration PDF Titration Chemistry Limitations To Titration titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. students who conduct a titration experiment may believe their results are as accurate. Limitations To Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Limitations To Titration A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. 1.1 introduction to titration. method validation of a titration ensures that the selected titration method and parameters will provide a. Limitations To Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Limitations To Titration 1.1 introduction to titration. The lack of a strong base titrant for the. the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. students who conduct a titration experiment may believe their results are as accurate as possible, but like any experiment, titration experiments. Limitations To Titration.
From chemistnotes.com
Precipitation Titration Principle, Types, and 5 Reliable Applications Limitations To Titration students who conduct a titration experiment may believe their results are as accurate as possible, but like any experiment, titration experiments contain. 1.1 introduction to titration. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. A titration is a quantitative, volumetric procedure used in analytical. Limitations To Titration.
From www.youtube.com
MOHR'S METHOD PRECIPITATION TITRATION YouTube Limitations To Titration a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. The lack of a strong base titrant for the. 1.1 introduction to titration. students who conduct a titration experiment may believe their results are as accurate as possible, but like any experiment, titration experiments contain. . Limitations To Titration.
From www.slideserve.com
PPT Non aqueous Titration theory and principles ppt PowerPoint Limitations To Titration method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. the aim of this booklet is to explore titration from a historical, theoretical and. Limitations To Titration.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Limitations To Titration students who conduct a titration experiment may believe their results are as accurate as possible, but like any experiment, titration experiments contain. 1.1 introduction to titration. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. The lack of a strong base titrant for the. . Limitations To Titration.
From byjus.com
Why is titration used? Limitations To Titration a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The lack of a strong base titrant for the. 1.1 introduction to titration.. Limitations To Titration.